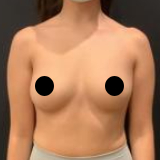
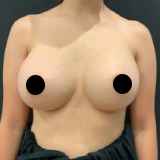
Allergan recently recalled their Biocell textured breast implants and tissue expanders. The recall was a result of ongoing studies to evaluate a rare lymphoma (Breast Implant Associated Anaplastic Large Cell Lymphoma, BIA-ALCL).
World-wide 573 cases of BIA-ALCL have been reported and 481 of these cases were patients that had Allergan Biocell textured implants. In the United States alone over 250,000 breast augmentations are performed every year. The incidence BIA-ALCl is extremely rare, however with the increase risk of BIA-ALCL in patients with Biocell textured implants the decision was made to recall the breast implants.
The recall is only for Allergan Biocell textured implants. This does not apply to Allergan smooth breast implants. It also does not include textured implants from the other breast implant manufactors, Sientra or Mentor. Allergan Biocell textured implants currently represent 5% of the breast implants used in the United States and all textured implants currently make up 10% of the implants used the U.S. To date there is currently no confirmed reports of BIA-ALCL in smooth breast implants.
It is important to understand that Biocell texturing is the shell of the implant. It does not refer to the interior of the implant. There is a misconception that gummy bear breast implants are all textured or shaped (tear drop) implants, and that is not true. A patient can have a smooth round gummy bear breast implant (highly cohesive gel). In fact, smooth round highly cohesive gel implants are much more common than textured cohesive gel implants.
The FDA and other health authorities currently do not recommend removal of the breast implants in patients that are asymptomatic. The most common symptoms are swelling and persistent pain. If you have any questions or concerns contact your plastic surgeon.
The recommendations for patients that have symptoms of persistent swelling or late on set swelling of their breast is evaluation for BIA-ALCL. The fluid around the breast implant can be sent for special testing to make the diagnosis of BIA-ALCL. If a patient is confirmed to have BIA-ALCL then surgical treatment is recommended. It is treated by removal of the breast implant and scar tissue surrounding the breast implant. When treated in a timely and effective mannor the outcomes and survival rates are extremely good.
It is important for patients to note that the incidence of this lymphoma is extremely rare. It is also important to know that presence of fluid around the breast implant does not mean that a patient has BIA-ALCL.
The press release has more information for patients on the current recommendations and process in place to further evaluate the safety of Biocell textured breast implants.
If you have any concerns about breast implants or questions about the type of breast implant that you currently have in place you should contact your plastic surgeon.